|
|
A
Snowflake Primer
... The basic facts about snowflakes and snow crystals ... |
|
|
Snowflakes
and snow crystals |
 Snowflakes and snow crystals are made of ice, and pretty
much nothing more. A snow
crystal, as the name implies, is a single crystal of ice. A snowflake
is a more general term; it can mean an individual snow crystal, or a few
snow crystals stuck together, or large agglomerations of snow crystals
that form "puff-balls"
that float down from the
clouds. Snowflakes and snow crystals are made of ice, and pretty
much nothing more. A snow
crystal, as the name implies, is a single crystal of ice. A snowflake
is a more general term; it can mean an individual snow crystal, or a few
snow crystals stuck together, or large agglomerations of snow crystals
that form "puff-balls"
that float down from the
clouds. |
|
The structure of crystalline
ice |
 The water molecules in an ice
crystal form a hexagonal lattice, as shown at right (the two structures
show different views of
the same crystal). Each red ball represents an oxygen atom, while
the grey sticks represent hydrogen atoms. There are two hydrogens
for each oxygen, so the chemical formula is
H2O. The six-fold symmetry of snow crystals ultimately
derives from the six-fold symmetry of the ice crystal lattice. The water molecules in an ice
crystal form a hexagonal lattice, as shown at right (the two structures
show different views of
the same crystal). Each red ball represents an oxygen atom, while
the grey sticks represent hydrogen atoms. There are two hydrogens
for each oxygen, so the chemical formula is
H2O. The six-fold symmetry of snow crystals ultimately
derives from the six-fold symmetry of the ice crystal lattice.
|
|
Snowflakes grow from water
vapor |
Snowflakes are not frozen raindrops. Sometimes
raindrops do freeze as they fall, but this is called sleet. Sleet particles
don't have any of the elaborate and symmetrical patterning found in snow crystals. Snow crystals form
when water vapor condenses directly into ice, which happens in the clouds.
The patterns emerge as the crystals grow. |
|
The simplest snowflakes |
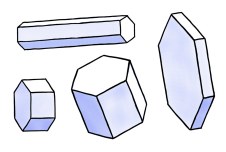  The most basic form of a snow crystal is a hexagonal prism, shown
in several examples at right. This structure occurs because certain
surfaces of the crystal, the facet surfaces, accumulate
material very
slowly (see Crystal
Faceting for more details).
The most basic form of a snow crystal is a hexagonal prism, shown
in several examples at right. This structure occurs because certain
surfaces of the crystal, the facet surfaces, accumulate
material very
slowly (see Crystal
Faceting for more details).
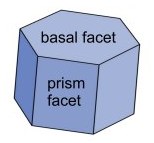
A hexagonal prism includes two hexagonal
"basal" faces and six rectangular "prism" faces, as shown in the
figure. Note that a hexagonal prism can be plate-like or
columnar, depending on which facet surfaces grow most quickly.
When snow crystals are very small, they
are mostly in the form of simple hexagonal prisms. But as they
grow, branches sprout from the corners to make more complex shapes.
Snowflake Branching describes how this happens. |
|
The Morphology Diagram |
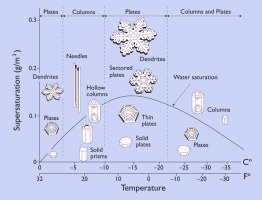 By growing snow crystals in the laboratory under
controlled conditions, one finds that their shapes depend on
the temperature and humidity. This behavior is summarized in the "morphology
diagram," shown at left, which gives the crystal shape under different conditions.
Click on the picture for a closer view. By growing snow crystals in the laboratory under
controlled conditions, one finds that their shapes depend on
the temperature and humidity. This behavior is summarized in the "morphology
diagram," shown at left, which gives the crystal shape under different conditions.
Click on the picture for a closer view.
The morphology diagram tells us a
great deal about what kinds of snow crystals form under what conditions.
For example, we see that thin plates and stars grow around -2 C (28 F),
while columns
and slender needles appear near -5 C (23 F). Plates and stars again
form near -15 C (5 F), and a combination of plates and columns are made around
-30 C (-22 F).
Furthermore, we see from the diagram that snow crystals
tend to form simpler shapes when the humidity (supersaturation) is low,
while more complex shapes at higher humidities. The most extreme
shapes -- long needles around -5C and large, thin plates around -15C -- form when the
humidity is especially high.
Why snow crystal
shapes change so much with temperature remains something of a scientific
mystery. The growth depends on exactly how water vapor molecules
are incorporated into the growing ice crystal, and the physics behind
this is complex and not well understood. It is the subject of
current research in my lab and elsewhere. |
|
The life of a snowflake |
The story of a
snowflake begins with water vapor in the air. Evaporation from
oceans, lakes, and rivers puts water vapor into the air, as does
transpiration from plants. Even you, every time you exhale, put
water vapor into the air.
When you take a parcel of air and cool it down, at some point the
water vapor it holds will begin to condense out. When this happens
near the ground, the water may condense as dew on the grass. High
above the ground, water vapor condenses onto dust particles in the air.
It condenses into countless minute droplets, where each droplet contains
at least one dust particle. A cloud is nothing more than a huge
collection of these water droplets suspended in the air.
In the winter, snow-forming clouds are still mostly made of liquid
water droplets, even when the temperature is below freezing. The
water is said to be supercooled, meaning simply that it is cooled
below the freezing point. As the clouds gets colder, however, the
droplets do start to freeze. This begins happening around -10 C
(14 F), but it's a gradual process and the droplets don't all freeze at
once.
If a particular droplet freezes, it becomes a small particle of ice
surrounded by the remaining liquid water droplets in the cloud.
The ice grows as water vapor condenses onto its surface, forming a
snowflake in the process. As the ice grows larger, the remaining
water droplets slowly evaporate and put more water vapor into the air.
Note what happens to the water -- it evaporates from the water
droplets and goes into the air, and it comes out of the air as it
condenses on the growing snow crystals. As the snow falls there is
a net flow of water from the liquid state (cloud droplets) to the solid
state (snowflakes). This rather complicated chain of events is how
a cloud freezes. |
|
The rest of the story |
| Alas, there's so much
more to the story -- it simply cannot fit here on a single page.
Snowflakes are fascinating objects (in my humble opinion), and you can
learn all kinds of interesting things about them in The Snowflake:
Winter's Secret Beauty. Click
here to see what's inside this book. |
|
The Science |
| If you want to see the
scientific aspects of snow crystal growth, I recommend a
review paper I recently wrote for the journal Reports on Progress
in Physics. |
For the answers to some common questions, like
Why do snow
crystals grow into such symmetrical forms?
and
Why is snow white?
continue on to the Snow Crystal Frequently Asked
Questions
page.... And there's a whole
separate page for that timeless question:
Is it really
true that no two snowflakes are alike? |
|
