|
|
Snowflake
Designer's Page
... Engineering the perfect snowflake ... |
|
Build a
better snowflake, and the world will shovel a path to your door. --KGL |
| A number of researchers have grown snow
crystals in the laboratory using several different methods. Some of these methods
are described here, along with pictures of the resulting synthetic snow crystals. |
| Free-fall
Growth |
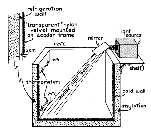  The simplest technique for growing snow crystals is to allow the crystals to fall freely
in a cold chamber. The images at right show a sketch of such a chamber, and few
sample crystals growth with this method [1]. Many additional images can be found in Free-falling
Snow.
The simplest technique for growing snow crystals is to allow the crystals to fall freely
in a cold chamber. The images at right show a sketch of such a chamber, and few
sample crystals growth with this method [1]. Many additional images can be found in Free-falling
Snow.
Since cold air is denser than warm air, the air in the cold chamber doesn't
mix much with the outside air, even with the top cover removed. To supersaturate the
air one needs only to breathe into the box, or use a humidifier, to produce a dense
fog. Within this cloud the water vapor pressure is then roughly equal to the water
saturation pressure, which is supersaturated about 5-15 percent relative to the ice
saturation vapor pressure (see Ice Properties).
The droplets do not spontaneously freeze until the temperature approaches -40 C, so ice
crystals must be nucleated in the cloud. Various smokes will provide suitable
nucleation sites, and dropping a small pellet of dry ice (which cools the air right around
it to -60 C) will also produce a fine cloud of sparkling ice crystals.
The tiny ice crystals grow rapidly in the supersaturated air, and fall very
slowly since their terminal velocity in air is low. As they grow larger, they fall
faster, and typically reach the chamber floor no larger than 0.1 mm. Large numbers
of crystals can be formed in this way. In spite of their small size, with a good
microscope a wide variety of morphologies can be observed using this simple technique.
|
| Growth
in a Moving Air Column |
A variation on the
free-fall technique is to use a moving column of air, adjusting the air flow speed to
equal the crystal's terminal velocity, so that it remains suspended as it grows [2]. By
using a tapered column the resulting air flow tends to stabilize the crystals in the
horizontal direction. This technique provides a very close match to natural snowfall, and
growth times of up to 30 minutes have been demonstrated. Since a growing crystal
exhibits substantial random motion in the moving airstream, each crystal must be removed
from the growth chamber for observation. Not surprisingly, snow crystals grown
using this technique bear an excellent resemblance to natural snow crystals. |
| Growth
on a Filament |
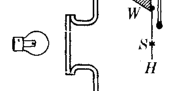 To grow larger snow crystal under better controlled conditions, it is necessary to support
the crystal as it grows. A thin filament is an obvious solution to the support
problem, and Nakaya used various filaments to grow the first artificial snow crystals in
the 1930's. Nakaya used a convection chamber, which is shown in the image at
right. Warm water produced water vapor at the bottom of the chamber, which was
carried up to the growth region by convection. Convection produces a somewhat
erratic airflow, and the filament does interfere with the crystal growth, but nevertheless
Nakaya was able to use this technique with great success (see Photo Collections).
To grow larger snow crystal under better controlled conditions, it is necessary to support
the crystal as it grows. A thin filament is an obvious solution to the support
problem, and Nakaya used various filaments to grow the first artificial snow crystals in
the 1930's. Nakaya used a convection chamber, which is shown in the image at
right. Warm water produced water vapor at the bottom of the chamber, which was
carried up to the growth region by convection. Convection produces a somewhat
erratic airflow, and the filament does interfere with the crystal growth, but nevertheless
Nakaya was able to use this technique with great success (see Photo Collections).
It's interesting to note that Nakaya realized his best snow crystals using a
stretched rabbit hair filament (he also tried spider web and other exotic materials).
The advantage of rabbit hair was that crystals tended to nucleate at only a few
places along the hair, so fairly isolated crystals could be grown. I tried this a
few times, but never with any real success -- wrong kind of rabbit, perhaps! |
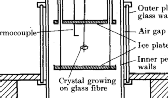 A
great advance introduced by Mason and collaborators was the use of a water vapor diffusion
chamber, which is shown at right [3] (see Designer
Snowflakes). The diffusion chamber has warm moist air at the top, just the
opposite of the convection chamber. The water vapor diffuses down from the top,
producing cool supersaturated air in the middle of the chamber. Since the chamber is
warm on top and cold on the bottom, convection is suppressed, resulting in very stable
conditions for snow crystal growth. A
great advance introduced by Mason and collaborators was the use of a water vapor diffusion
chamber, which is shown at right [3] (see Designer
Snowflakes). The diffusion chamber has warm moist air at the top, just the
opposite of the convection chamber. The water vapor diffuses down from the top,
producing cool supersaturated air in the middle of the chamber. Since the chamber is
warm on top and cold on the bottom, convection is suppressed, resulting in very stable
conditions for snow crystal growth.
The vertical temperature gradient in the diffusion chamber can also be put to
advantage, as was shown by Mason et al. If a long string, such as a piece of nylon
fishing line, is hung down the center of the chamber, then ice crystals will grow all
along the string. In this way one can immediately observe the different growth
morphologies as a function of growth temperature (see the Snowflake Primer). |
| Growth
on a Substrate |
A number of workers studying
snow crystal growth have made observations of crystals grown on a substrate.
Although the substrate definitely perturbs the growth to some extent, the effects are not
too bad if the supersaturation is low and the substrate is clean. In this case
crystals will not spontaneously nucleate on the substrate, and thus isolated samples can
be observed.
Some of the best pictures were obtained by Gonda and coworkers [4], who
developed a technique by which snow crystals are grown directly on a sapphire
window. Supersaturated air is produced in a growth chamber, and silver iodide smoke
is introduced to nucleate the production of snow crystals. The crystals grow for a
bit while suspended in the air, then fall onto the window, where further growth can be
photographed. The technique clearly produces nice symmetrical snow crystals (see the
examples below), which can be observed using a microscope objective positioned directly
underneath the substrate for high-resolution imaging. The technique seems to work
best for plate-like crystals and low supersaturations, where the perturbations from the
substrate are minimal. |
   
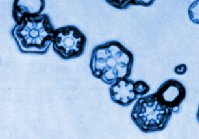
Above are samples of snow crystals grown on a substrate, published by T. Gonda and
coworkers in the Journal of Crystal Growth [4].
|
| Growth
of Electrodynamically Levitated Crystals |
 A novel approach to producing artificial snow crystals is to levitate the
growing crystals in an electrodynamic trap (a Paul-type ion trap). Such a scheme produces
isolated single crystals, since the levitated crystal doesn't touch anything
directly. Growing large crystals using this technique is difficult, however, since
as the weight of the crystal increases it quickly becomes too heavy to support in the
trap. Electrodynamic traps for ice crystal growth were first demonstrated by B.
Swanson and collaborators [5], and more on this subject can be found at the Ice
Particle Microphysics Laboratory. A novel approach to producing artificial snow crystals is to levitate the
growing crystals in an electrodynamic trap (a Paul-type ion trap). Such a scheme produces
isolated single crystals, since the levitated crystal doesn't touch anything
directly. Growing large crystals using this technique is difficult, however, since
as the weight of the crystal increases it quickly becomes too heavy to support in the
trap. Electrodynamic traps for ice crystal growth were first demonstrated by B.
Swanson and collaborators [5], and more on this subject can be found at the Ice
Particle Microphysics Laboratory.
|
| Growth
on Ice Needles |
 Our favorite technique for growing snow crystals is to grow them on the ends of long ice
needles, and many examples can be found in our (see Designer
Snowflakes). This method was first applied by Bartlett, van den Heuval, and
Mason in 1963 [6], when they discovered that ice crystals growing under the influence of a
large applied voltage developed into long thin ice needles (for details of this see Electric
Growth).
Our favorite technique for growing snow crystals is to grow them on the ends of long ice
needles, and many examples can be found in our (see Designer
Snowflakes). This method was first applied by Bartlett, van den Heuval, and
Mason in 1963 [6], when they discovered that ice crystals growing under the influence of a
large applied voltage developed into long thin ice needles (for details of this see Electric
Growth).
We have found that electric needles grown along
the c-axis are wonderfully well suited for producing isolated snow crystals, particularly
large stellar crystals. By first growing a single electric needle to a length of ~1 cm,
subsequent crystal growth at the end of the needle is quite unperturbed by the underlying
support. Also the electric needles are thin and strong, and hold a growing snow crystal
quite rigidly for sharp photography. |
| Growth
under Unusual Conditions |
Nearly all the work on snow crystal growth done to date has been under normal
atmospheric conditions, i.e. with standard atmospheric pressure and standard atmospheric
constituents. Growth under different background pressures and in different gases has
been realized, however, and with interesting results. Under higher pressures, for
example, the diffusion constant decreases, leading to "enhanced" growth
morphologies -- thinner plates and longer needles than occur under normal
conditions. There is also evidence that the kinetic growth coefficients depend on
background gas.
Finally, a number of workers (including the author) have found that trace
chemical impurities in the background air can greatly affect snow crystal growth, a topic
which has not been well studied to date. |
|
[1] V. J. Schaefer and J. A. Day, Peterson
Field Guides: Atmosphere (Houghton Mifflin, 1981).
[2] T. Takahashi and N. Fukuta, J. Meteor. Soc. Japan 66,
841 (1988); T. Takahashi, T. Endoh, G. Wakahama, and N Fukuta, J. Meteor. Soc. Japan
69, 15 (1991).
[3] B. Mason, in The Physics of Clouds (Oxford University Press, 1971).
[4] T. Gonda, S. Nakahara, and T. Sei, J. Cryst. Growth 99,
183 (1990); T. Gonda and S. Nakahara, J. Cryst. Growth 160, 162
(1996); T. Gonda and S. Nakahara, J. Cryst. Growth 173, 189
(1997)].
[5] B. D. Swanson, N. Bacon, E. J. Davis and M. B. Baker, Q. J. Roy. Meteor.
Soc. 125, 1039 (1999).
[6] J. T. Bartlett, A. P. van den Heuval, B. J. Mason, Z. angue. Math. Phys.
14, 509 (1963). |
|
