| Physical
Properties of Ice |
|
Crystalline Structure of Ice.
Ice can assume a large number of different
crystalline structures, more than any other known material. At ordinary pressures
the stable phase of ice is called ice I, and the various high-pressure phases of ice
number up to ice XIV so far. (Ice IX received some degree of notoriety from Kurt
Vonnegut's novel Cat's Cradle.)
There are two closely related variants of ice I: hexagonal ice Ih, which has
hexagonal symmetry, and cubic ice Ic, which has a crystal structure similar to
diamond. Ice Ih is the normal form of ice; ice Ic is formed by depositing vapor at
very low temperatures (below 140°K). Amorphous ice can be made by depositing
water vapor onto a substrate at still lower temperatures.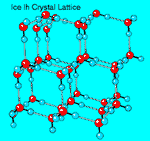
Each oxygen atom inside the ice Ih lattice is surrounded by four other oxygen
atoms in a tetrahedral arrangement. The distance between oxygens is approximately
2.75 Angstroms. The hydrogen atoms in ice are arranged following the
Bernal-Fowler rules: 1) two protons are close (about 0.98A) to each oxygen atom,
much like in a free water molecule; 2) each H20 molecule is oriented so that
the two protons point toward two adjacent oxygen atoms; 3) there is only one proton
between two adjacent oxygen atoms; 4) under ordinary conditions any of the large number of
possible configurations is equally probable. |
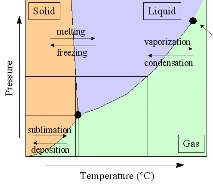 Phase Diagram of Water and
Ice. The plot at right shows the phase diagram of water (click on
the image for an expanded version). The triple point of water --
when ice, water, and water vapor can coexist -- is at a temperature of 0.01C (0C =
273.16K), and a pressure of 6.1 mbar. Water is the only substance which we commonly
experience near its triple point in everyday life. Phase Diagram of Water and
Ice. The plot at right shows the phase diagram of water (click on
the image for an expanded version). The triple point of water --
when ice, water, and water vapor can coexist -- is at a temperature of 0.01C (0C =
273.16K), and a pressure of 6.1 mbar. Water is the only substance which we commonly
experience near its triple point in everyday life.
|
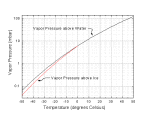 Equilibrium Vapor
Pressure of Ice and Water. The plot at right shows the equilibrium
water vapor pressure of ice and water as a function of temperature, over the range of
interest for snow crystal growth [1]. The pressure units are in mbar, and one can
convert to other units using a conversion calculator (1 mbar = 100 Pascal (Newtons/square
meter) = 0.75 mm Hg = 0.001 atmospheres.) Equilibrium Vapor
Pressure of Ice and Water. The plot at right shows the equilibrium
water vapor pressure of ice and water as a function of temperature, over the range of
interest for snow crystal growth [1]. The pressure units are in mbar, and one can
convert to other units using a conversion calculator (1 mbar = 100 Pascal (Newtons/square
meter) = 0.75 mm Hg = 0.001 atmospheres.)
The vapor pressure is well described by the Clausius-Clapeyron relation, and
a fit to the data yields the approximations:
Pwater(T) = [2.8262e9 - 1.0897e6*T - 94934*T2
+ 582.2*T3]exp(-5450/TK)
Pice(T) = [3.6646e10 - 1.3086e6*T - 33793*T2]exp(-6150/TK)
where pressures P are in mbar, the temperature T is in
degrees Celsius, and TK is in degrees Kelvin (Note 0C = 273.16K). These
approximate expressions are accurate to better than 0.1 percent from -50C to 50C.
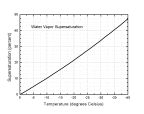 The plot
at the right shows the water vapor supersaturation value, equal to (Pwater-Pice)/Pice.
This is the supersaturation level that is typically found in dense clouds, which
after all are made of water droplets. Supersaturation levels higher than this are
probably quite unusual in the atmosphere. The plot
at the right shows the water vapor supersaturation value, equal to (Pwater-Pice)/Pice.
This is the supersaturation level that is typically found in dense clouds, which
after all are made of water droplets. Supersaturation levels higher than this are
probably quite unusual in the atmosphere. |
Various constants
related to Ice and the Formation of Snow Crystals.
Mass of a water molecule:
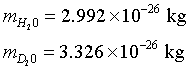 |
Ice density (near 0°C):
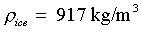 |
Latent heats of sublimation, evaporation,
and melting:
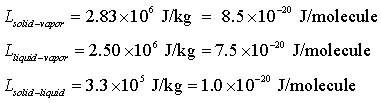 |
Heat capacity of ice, water (near 0°C):
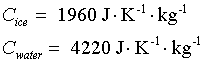 |
Electric dipole moment of a water
molecule:
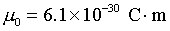 |
Intrinsic dielectric polarizability of a
water molecule:
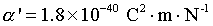 |
Total dielectric polarizability of a
water molecule (near 0°C):
 |
Ice surface energy:
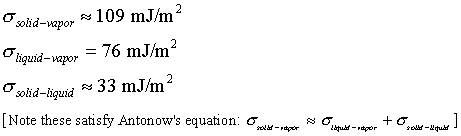 |
Diffusion constant for water molecules in
air at STP:
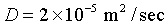 |
Critical radius for nucleation:
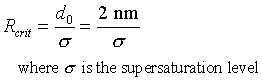 |
Coefficient of thermal expansion of ice:
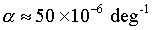 |
Thermal conductivity of ice (near -20°C):
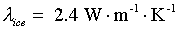 |
|
| [1] From B. J. Mason, The Physics of Clouds
(Clarendon Press, 1971).. |
