|
|
Snowflake
Branching
... The origin of the complex structure of snowflakes ... |
|
| One thing you notice right away about
snow crystals is that they form some elaborate and complex shapes -- often displaying
lacy, branching structures. Where does this complexity come from? After all,
snow crystals are nothing more than ice which has condensed from water vapor. How
does the simple act of water vapor freezing into ice produce such intricate designs? |
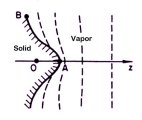 The answers to these questions lie in
just how water molecules travel through the air to condense onto a growing snow crystal.
The water molecules have to diffuse through the air to reach the crystal,
and this diffusion slows their growth. The farther water molecules have to diffuse
through the air, the longer it takes them to reach the growing crystal. The answers to these questions lie in
just how water molecules travel through the air to condense onto a growing snow crystal.
The water molecules have to diffuse through the air to reach the crystal,
and this diffusion slows their growth. The farther water molecules have to diffuse
through the air, the longer it takes them to reach the growing crystal.
So consider a flat ice surface that is growing in the air. If a small
bump happens to appear on the surface, then the bump sticks out a bit farther than the
rest of the crystal. This means water molecules from afar can reach the bump a bit
quicker than they can reach the rest of the crystal, because they don't have to diffuse
quite as far.
With more water molecules reaching the bump, the bump grows faster. In
a short time, the bump sticks out even farther than it did before, and so it grows even
faster. We call this a branching instability -- small bumps develop
into large branches, and bumps on the branches become sidebranches. Complexity is
born. This instability is a major player in producing the complex shapes of snow
crystals. |
 When the branching instability
applies itself over and over again to a growing snow crystal, the result is called an ice dendrite.
The word dendrite means "tree-like," and stellar dendrite snow crystals
are common (see the Guide to Snowflakes). When the branching instability
applies itself over and over again to a growing snow crystal, the result is called an ice dendrite.
The word dendrite means "tree-like," and stellar dendrite snow crystals
are common (see the Guide to Snowflakes).
We can change diffusion in the lab and see how dendrites change. If one
grows snow crystals in air below atmospheric pressure, they have fewer branches.
This is because diffusion doesn't limit the growth so much at lower air pressures, so the
branching instability is not so strong. At higher pressures, more branches appear.
|
The growth of snow
crystals depends on a balance between faceting (see Crystal
Faceting) and branching. Faceting tends to make simple flat surfaces,
while branching tends to make more complex structures. The interplay between
faceting and branching is a delicate one, depending strongly on things like temperature
and humidity. This means snow crystals can grow in many different ways, resulting in
the great diversity we see in snow crystal forms. |
|
|
