Research
ePetri Dish Project
The ePetri dish is a chip-scale lensfree microscopy platform that can automatically perform high resolution (~0.66 micron) microscopy imaging over a large field-of-view (6mm × 4mm). Unlike the other lensless microscopy methods, this new approach is fully capable of working with cell cultures or any samples in which cells/bacteria may be contiguously connected, and thus, it can significantly improve Petri dish based cell/bacteria culture experiments. With this approach providing a compact, low-cost and disposable microscopy imaging solution, we can start to transit Petri dish based experiments from the traditionally labor-intensive process to an automated and streamlined process. This technological shift from an inert petri-dish to an ePetri dish platform is appropriately timely as well, because, the cost of high performance CMOS imaging sensors (which are widely used in cellphone cameras and webcams) have recently reached a price point where they can be used as recyclable or disposable components.
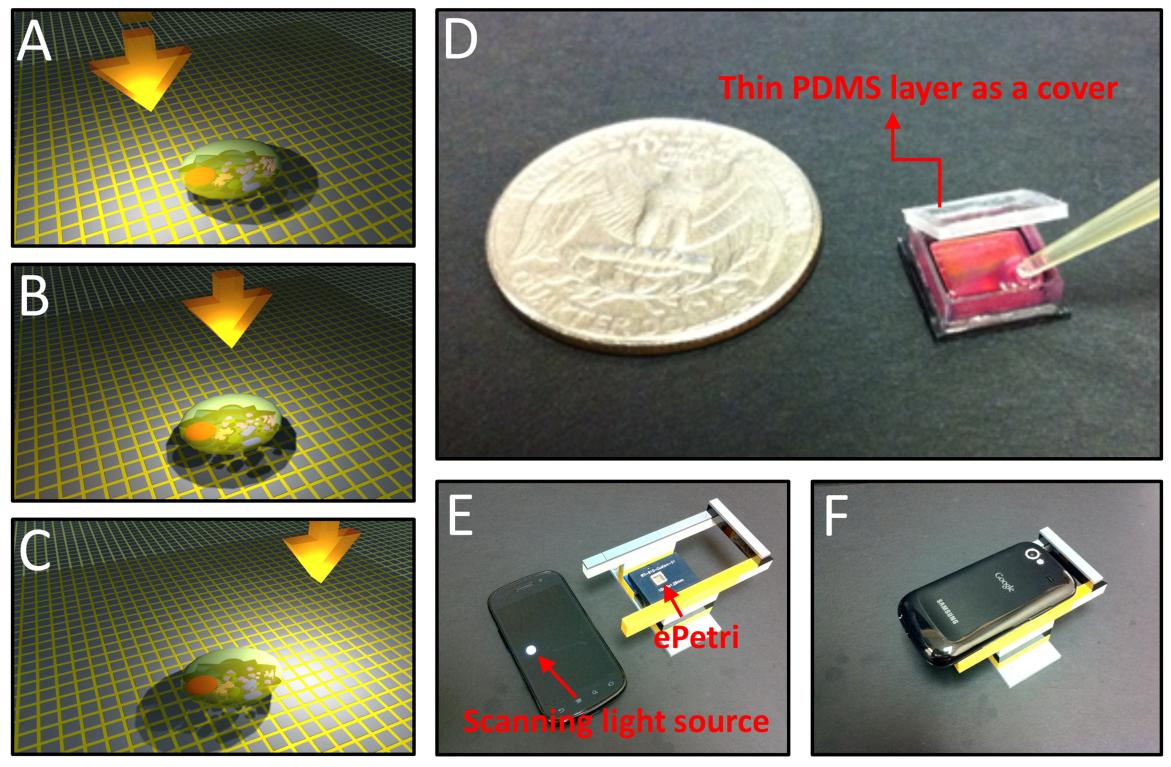
Fig. 1 ePetri dish Platform. (A-C) With the incremental tilt/shift
of the illumination, the target cells' shadow will incrementally
shift across the sensor pixels. These sub-pixel shifted low
resolution images will be used to reconstruct the high resolution
image by using pixel super resolution algorithm. (D) The ePetri prototype. A thin PDMS layer is used as a cover to prevent the evaporation of the culture media while allowing for CO2 exchange between the well and exterior. (E-F) The ePetri imaging
platform. We used the LED screen of a smartphone as the
scanning light source. The holder is built with Lego blocks.
Conceptually, this method of microscopy imaging is simple to understand. We simply culture cells or place cells of interest directly on the surface of a CMOS image sensor. We sequentially tilt/shift an incoherent illumination source above the sample and acquire a sequence of raw images. With the incremental tilt/shift of the illumination, the target cells' shadow will incrementally shift across the sensor pixels (Fig. 1(A-C)). The amount of shadow shift is proportional to the passivation layer thickness and the tilt/shift extent of the light source. As long as the shadow shift between each raw image frame is much smaller than the physical pixel size, we can then combine the information from multiple sub-pixel-shifted low resolution shadow images to create a single high resolution image with a pixel super-resolution algorithm.
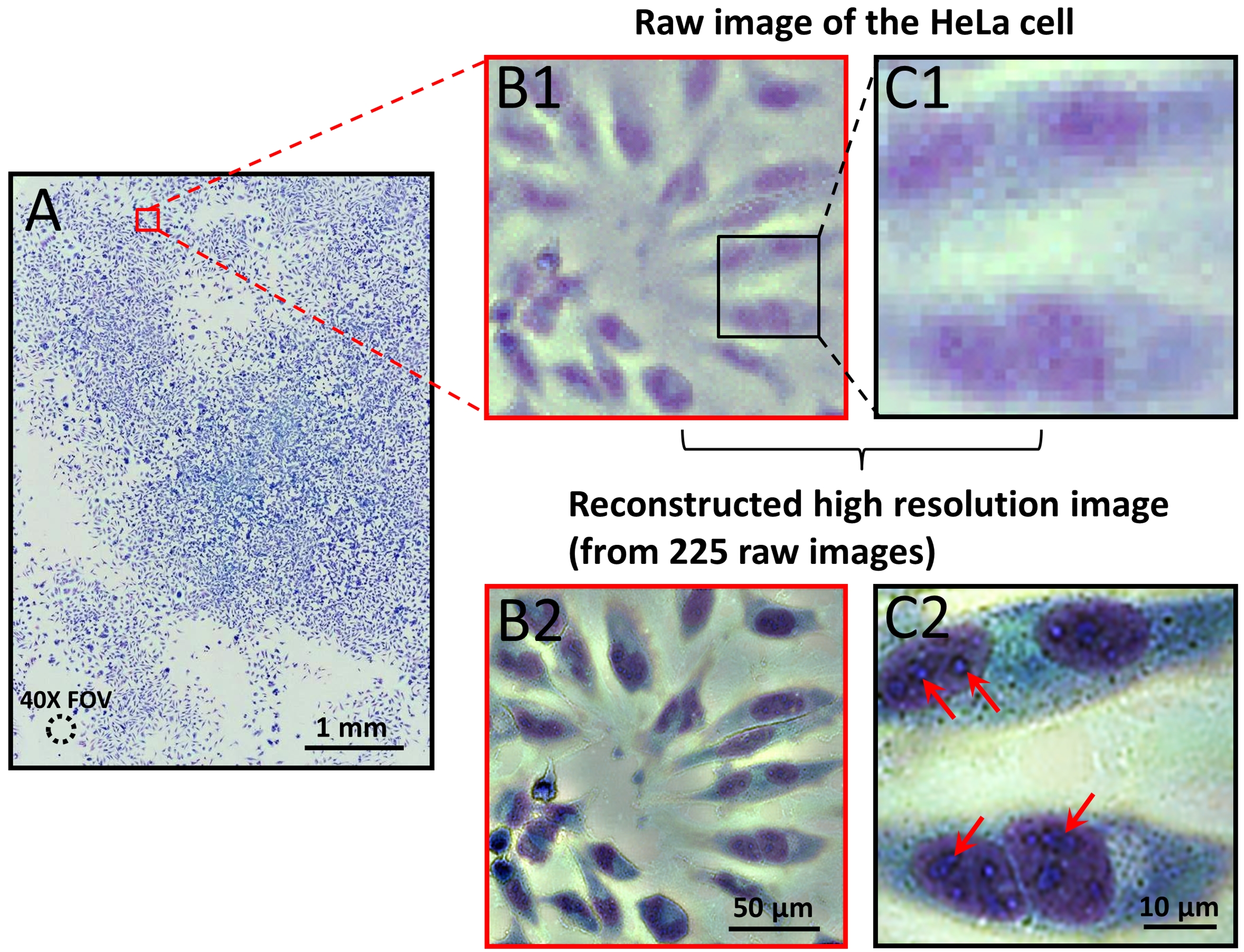
Fig. 2 (A) Large-field-of-view color imaging of the confluent
cell sample. The field of view of a 40X objective lens is also shown
in left bottom. (B1) and (C1), raw images of a small region
of (A). (B2) and (C2), the reconstructed high resolution images corresponding to (B1) and (C1).
Fig. 2(A) shows the reconstructed color image of the confluent HeLa cell sample. The amount of details in the reconstructed color image is too large to fully display on a computer screen or print on a printer; we have provided vignette views of selected regions for comparison in Fig. 2. Fig. 2(B1) and (C1) shows the raw images from a small region of Fig. 2(A). Fig. 2(B2) and (C2) shows the corresponding reconstructed high resolution image of (B1) and (C1). From the reconstructed high resolution image in Fig. 2(B2) and (C2), we can readily discern organelles within the HeLa cell, such as multiple nuclear granules (indicated by red arrows), and the nucleus.
The ePetri dish is a platform technology. Since the top surface of the sensor chip is unmodified, a user is free to build upon it. It is very possible to simply use the ePetri as an imaging platform for a large number of sophisticated lab-on-a-chip designs, such as microorganisms detection based on the use of closed dielectrophoretic cages, droplet-based platforms for cell encapsulation and screening, microfluidics-based phenotyping imaging and screening of multicellular organisms, and high throughput malaria infected erythrocyte separation and imaging. It is also possible to modify the ePetri dish to serve as a self-contained incubator and imaging unit.
ePetri Inc. website
Related Video

References
Guoan Zheng, Seung Ah Lee, Yaron Antebi, Michael B. Elowitz, and Changhuei Yang;
“The ePetri dish, an on-chip cell imaging platform based on subpixel perspective sweeping microscopy (SPSM)” PNAS, 2011 (published ahead of print October 3, 2011, doi:10.1073/pnas.1110681108)(pdf).
|