Background and Rationale
Hematopoietic Stem Cells (HSCs) can give rise to a large variety of cells.
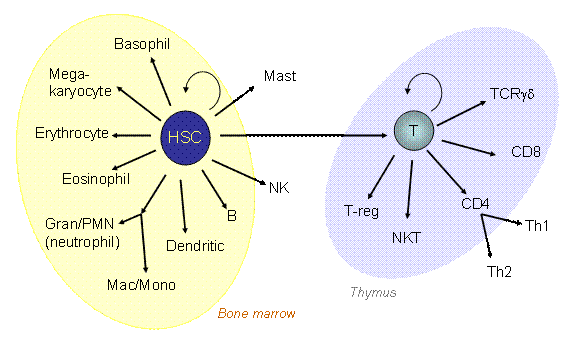
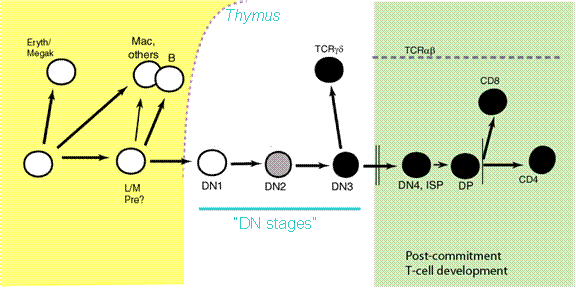
We are interested in the process by which HSCs differentiate into T cells. This process occurs after uncommitted blood precursor cells enter the Thymus, and requires environmental signals provided by the thymus. Fate selection can take up to 10-12 cell cycles to be completed, and occurs through steps in which at least four developmental stages can be distinguished by cell-surface phenotype. By convention, cells at these stages are called DN1 – DN4.
Genetic research based on gene knockouts in mice has led to the identification of several key transcriptional regulators and signaling events. All of these regulators are required to generate committed T-cell precursors. However, most of these regulators are also required in different combinations or levels of activity for other cell fates. There is no single “master regulator” of T-cell differentiation.
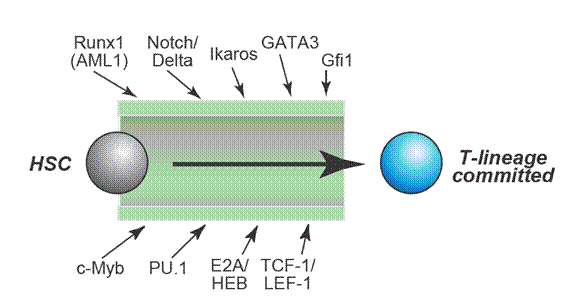
The one environmental signal that is rate-limiting and indispensable for T-cell specification is Notch-Delta signaling. Notch signals are required throughout the DN stages of T cell development and is guaranteed by expression of the Notch ligands Delta-like 4 and Delta-like 1 in the thymic stroma.
The time windows for the activities of these factors overlap throughout the early stages of T-cell development. Many of them are required both within the thymus and in the prethymic stages, to generate T-competent precursors.
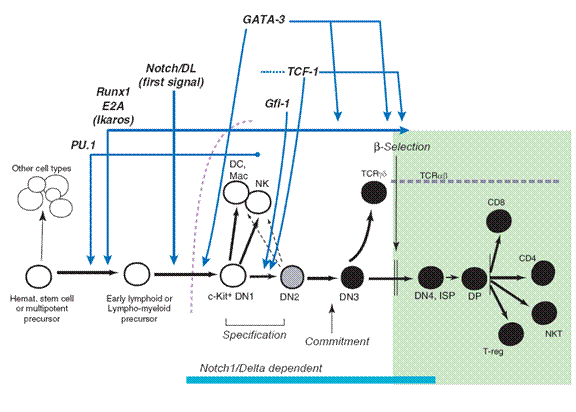
But the exact sequence of positive and negative regulatory events that are necessary and sufficient for T cell development remain a mystery.
Major Issues:
The decision between a T cell fate and alternative fates is decided remarkably late in this process. Commitment occurs at the DN3(a) stage, long after the cells in any particular cohort have been differentiating in the thymus and responding to Notch-Delta signals.
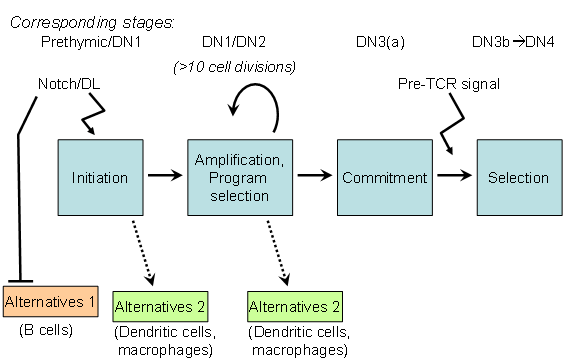
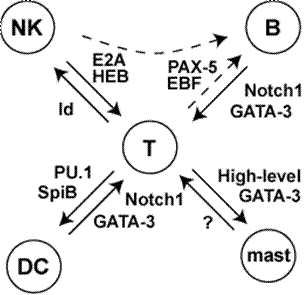 A
key objective of this project is to understand the full basis of
plasticity that the cells retain until this point of
commitment.
The project is made possible by dramatic results of perturbation
experiments that reveal the ability of single transcription factors to
alter the developmental identity of the cells, even at the DN3a stage,
if they are expressed at elevated levels. This provides a
point
of entrée into the network establishing T-cell identity.
A
key objective of this project is to understand the full basis of
plasticity that the cells retain until this point of
commitment.
The project is made possible by dramatic results of perturbation
experiments that reveal the ability of single transcription factors to
alter the developmental identity of the cells, even at the DN3a stage,
if they are expressed at elevated levels. This provides a
point
of entrée into the network establishing T-cell identity.
Dr.
Rothenberg lecturing on early T-cell development