
Professor Johnson's group does research on non-equilibrium and metastable materials. During the past decade, they have developed unusual metallic alloys which fail to crystallize during solidification at low cooling rates, thus forming "bulk" glasses. Research on the liquid alloys includes fundamental studies of rheology, atomic diffusion, crystallization kinetics, liquid/liquid phase separation, and the glass transition. Research on the solid "glassy" materials includes studies of elastic properties, and mechanisms of deformation, flow, and fracture.
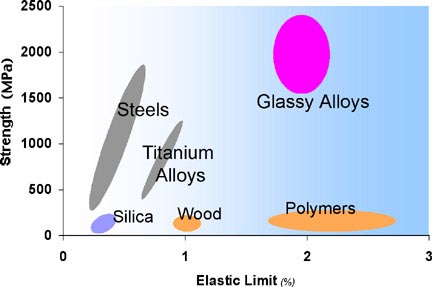
Typical strengths and elastic limits for various materials. Metallic
glasses are unique.
Conventional metallic materials have a crystalline structure consisting
of single crystal grains of varying size arranged in a microstructure.
Such structures are produced by the nucleation and growth of crystalline
phases from the molten alloy during solidification. By contrast, certain
oxide mixtures (e.g. silicate glasses), have such sluggish crystal nucleation
and growth kinetics, that the liquid can be readily undercooled far
below the melting point of crystals (e.g. a quartz crystal). At deep
undercooling, these oxide melts undergo a "glass transition"
and freeze as vitreous solids. Professor Johnson's group have developed
multicomponent metal alloys which vitrify with the same ease as observed
in silicate melts. These bulk metallic glasses (BMG's) have unusual
properties. They are typically much stronger than crystalline metal
counterparts (by factors of 2 or 3), are quite tough (much more so than
ceramics), and have very high strain limits for Hookean elasticity (see
figure above). A new class of engineering materials, BMG's offer an
opportunity to revolutionize the field of structural materials with
combinations of strength, ductility, toughness, and processability outside
the envelope achievable using current technology.
Return to top
For Additional Information, contact
www@matsci.caltech.edu









