| |
Research
Lab Tour
Publications
People
Gallery
Courses
Opportunities
Links
Contact
Home

|
We are working with nano-machines sensitive enough to interface with the world of proteins and biomolecules
 We are fabricating ultrasmall mechanical structures (e.g. 1.8 µm x 120 nm x 100 nm) We are fabricating ultrasmall mechanical structures (e.g. 1.8 µm x 120 nm x 100 nm) - These mechanical structures are made from fairly stiff mechanical materials like Silicon-Carbide or Silicon-Nitride
- These mechanical structures have high mechanical vibrational frequencies. Remember frequency ~ √(k/m) ~ 450 MHz in our case.
- Any material, like a protein molecule, added onto this structure will change the total mass of the resonator, and through that, the resonance frequency of the oscillation.
- By detecting this mechanical frequency shift, we can measure the mass of added species on our device.
- Our technology is at the point where we can detect and identify large protein molecules
Why is this important? Basically...
- It will help us identifying the proteins in a given cell, thus providing important information about the state of the cell (like if it is normal, cancerous, or have some other problems)
- It can be used as a tool to measure environmental contamination.
The first item above deserves a more detailed observation... See the bottom of the page for a fuller explanation on the importance and context of protein detection.
Ultimate goal...
- is to fabricate a compact mass spectrometer system...
- consisting of a densely-packed array of nanomechanical devices,
- interfaced with the cells on the front end through a microfluidics processor chip and nano-ESI
- interfaced with the outside world on the back end through on-chip integrated CMOS detection and amplification electronics
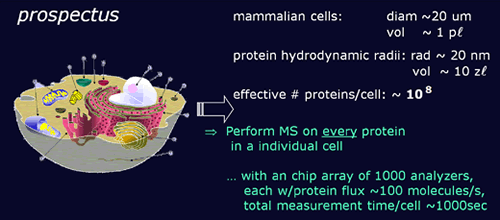
- so that the ultimate system will be able to analyze all the contents of a mammalian cell in 20 minutes.
What is the current state of art?
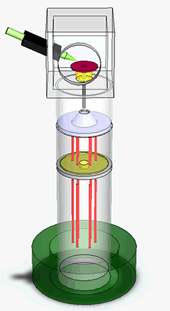
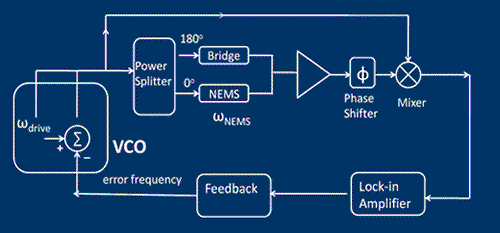
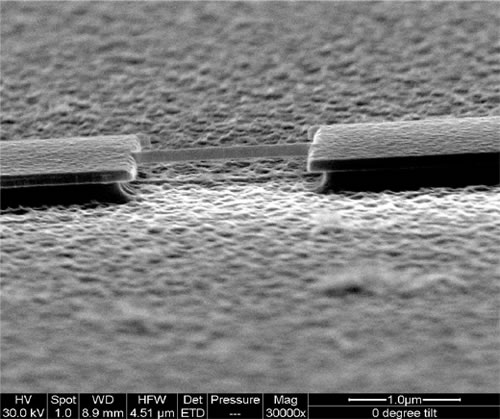
We have just finished performing a proof of principle experiment of the protein mass detection using a single NEMS device. The schematics of the experiment, the simplified circuit for the measurement and the device micrograph are shown in the figures. We use a commercial electrospray ionization system to produce the ionized protein in the gas phase. These proteins, generated at room temperature and atmospheric pressure, are then transported to the NEMS, located about 2 meters away, using a ion guiding system. The NEMS is placed about 5mm from the end of the ion guiding system. The NEMS temperature is maintained at 40K to ensure physioadsorprtion of the proteins. Each protein that lands on the NEMS produces a change in the resonant frequency. The change in frequency of the NEMS is a function of the mass of the protein and its position along the length of the NEMS. These changes in the resonant frequency of the NEMS are tracked using a phase locked loop.
You can read about our latest research in reference (1)
What are we working on right now?
We are working on making these devices even more sensitive, in a systematic, repeatable way. We are rushing to develop techniques to detect both position and mass of the added proteins. We are collaborating with LETI to fabricate an array of these devices on the same chip.
What advantages does our method have over other protein detection methods, namely mass-spectrometry?
Conventional mass spectrometry is a great technological success, cultivated through decades of scientific progress. Mass spectrometers are basically a family of instruments that measure electromagnetic properties of ionized bio-molecules thus determining their mass-to-charge ratios (unlike NEMS based MS). You can read about different kind of mass spectrometers and their working principles here: http://masspec.scripps.edu/
In order to make a useful measurement to obtain biologic information, mass spectrometers have a multitude of performance criteria, which includes for example: mass resolution, mass accuracy, detection limit, sample consumption, data acquisition rate, possession cost, operation cost, calibration requirement...
No mass spectrometer in existence is perfect. All of them have positive sides, as well as negative sides. Within this spectrum of instruments, our nano-mechanics based approach have the following unique capabilities:
- NEMS MS directly measures the mass of the proteins, not mass-to-charge ratios. Analysis of the data would be less equivocal.
- NEMS MS have a great dynamic range. It will have a sensitivity of single Dalton (reference 2) and an upper detection limit of 100s of MegaDaltons.
- It is possible to fabricate an array of NEMS mass spectrometers. This is helpful to prevent abundant proteins masking the presence of scarce proteins.
- NEMS MS can naturally be interfaced with a microfluidics front end and nano-scale electrospray ionization tips, that can effectively transport precious biological material, with minimal loss, on top of NEMS mass spectrometers.
- NEMS MS systems will be very cheap, disposable mass spectrometers.
Personnel
Adam Neumann, Eric Sage, and Warren Fon
References and Related Papers
- E. Sage, A. Brenac, T. Alava, R. Morel, C. Dupre, M.S. Hanay, M.L. Roukes, L. Duraffourg, C. Masselon, S. Hentz, Neutral particle mass specgtrometry with nanomechanical systems, 6482 (2015).
- M.S. Hanay, S. Kelber, A.K. Naik, D. Chi, S. Hentz, E.C. Bullard, E. Colinet, L. Duraffourg, M.L. Roukes, Single-protein nanomechanical mass spectrometryt in real time, Nature Nanotechnology, 7602-608 (2012)
- A. K. Naik, M. S. Hanay, W. K. Hiebert, X. L. Feng, M. L. Roukes, Towards single-molecule nanomechanical mass spectrometry, Nature Nanotechnology, 4, 445-450 (2009))
- K.L. Ekinci, X. M. H. Huang, M.L. Roukes, Ultrasensitive nano-electromechanical mass detection, Appl. Phys. Lett. 84 4469 May 2004
- Y. T. Yang, C. C. Callegari, X. X. L. Feng, K. K. L. Ekinci, M. M. L. Roukes, Zeptogram-scale nanomechanical mass sensing, Nano letters 6, 583 (2006).
- X.L.Feng, Ph.D. Thesis,”Ultra-High Frequency Nanoelectromecahnical Systems with Low-Noise Technologies for Single-Molecule Mass Sensing” August 14 2006 (2006).
- K.L. Ekinci, Y.T. Yang, and M.L. Roukes, Ultimate limits to inertial mass sensing based upon nanoelectromechanical systems 95, 2682 (2004).

|