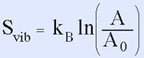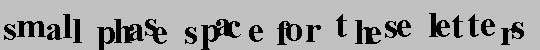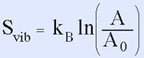
The idea behind vibrational entropy is that a "phase space" is explored by atoms in a solid as they vibrate. This phase space is spanned by momentum and position coordinates. The larger the phase space, the larger the vibrational entropy.
This is illustrated by the following two lines of text. The top line has configurational entropy, at least assuming that another similar line of text would be disordered differently. Suppose for a moment that the temperature is low, however. With a modest increase in temperature, the positions of these letters in the top line do not change. For the stationary letters in the top row, there is no absorbtion of heat when the temperature is increased.
On the other hand, the letters in the second row will wiggle even more if the temperature is raised, so they will absorb heat. The second line has the larger vibrational entropy, and this vibrational entropy will increase with temperature. The area, A, that is explored on your screen as the atoms move is related to the vibrational entropy, Svib, as: